It all started on a February day when I boarded a plane flying to Las Vegas for an interview at the University of Nevada, Las Vegas (UNLV) School of Dental Medicine. My flight took me through Minneapolis and on to the only location where “the sun goes down, but the city gets brighter.” After a short cab ride to the dental school, I was soon seated for an interview. Shortly after we began, Dr. Ron Lemon, graduate program director and endodontist, started talking about “regrowing pulps.” As a fourth-year dental student, I thought this was a trick. I smiled, nodded, and luckily received an offer to enroll in the pediatric residency program despite my ignorance.
My time in the sun allowed me to take advantage of everything UNLV’s program had to offer. Although they did not offer an endodontic residency, I quickly discovered that opportunities to perform endodontic therapy in children were plentiful. This sparked my interest in learning the properties of mineral trioxide aggregate and how to handle it clinically in order to best suit the needs of my patients.
It took me a few cases to learn how to handle the material, but quickly I realized that the procedures could all be performed using familiar instruments. I’d like to share the knowledge I learned during my time in the sun playing with sand, while holding an amalgam carrier in my hand.
PROPERTIES OF MINERAL TRIOXIDE AGGREGATE (MTA)
Mineral trioxide aggregate (MTA) is a bioactive material composed of dicalcium silicate, tricalcium silicate, tricalcium aluminate, and tetracalcium aluminoferrite. Bismuth oxide is added as an agent rendering the material radiopaque and giving it a thickness approximately equivalent to 7 mm of aluminum.1 This addition gives the material more radiopacity than dentin and gutta percha. As a result, MTA is readily detected upon radiographic examination and ensures proper placement of the material and allows for radiographic follow up.
When mixed with water, MTA begins to set and releases calcium ions, which in turn leads to the formation of hydroxy appetite as it combines with phosphate ions that are present in the body. This layer adheres to dentin through a chemical bond as the material sets and provides valuable sealing capabilities.2
The aggregate material is bacteriostatic due to its basic pH and has been shown to be an inhibitor of bacterial growth. Recent studies have shown that MTA has potential to entomb bacteria in dentinal tubules as it forms tag-like structures that seal off tubules. This action allows the material to provide a sealing function in many different clinical scenarios. 3,4
MTA can be successfully utilized in primary teeth for indirect and direct pulp caps, pulpotomies, root-end filling (apexification), and perforation repair. In permanent teeth, the uses are even more diverse as they include direct and indirect pulp caps, pulpotomies, root-end filling (apexification), regenerative endodontics, orthograde obturation, perforation repair, and apicoectomy. 5,6
ADVANTAGES
Faster Bridging Than Calcium Hydroxide
MTA has been shown to exhibit faster bridging qualities than calcium hydroxide. MTA forms a dentin bridge in a shorter period of time than calcium hydroxide. 7 The bridge is detectable radiographically once it is 0.5 mm in thickness. 8 The formation of more tooth structure underneath the material may help provide a better seal against bacterial leakage, which is a common etiology of endodontic failures.9
Bioactive
MTA has been shown to stimulate hard tissue formation in contact with vital pulp cells and periapical tissues. Properties of the material stimulate the formation of dentin, cementum, and the periodontal ligament. The capability of the material to be osteoinductive when used in different tissue environments accounts for MTA’s wide variety of uses in vital and non-vital pulp therapy in both permanent and primary teeth. 5
Biocompatible
Unlike calcium hydroxide, MTA does not induce a layer of coagulative necrosis which results in hard tissue formation. As noted above, MTA has been shown to be compatible with pulp and periapical tissues when routinely placed in contact with them during therapy. 7 Unintentional extrusion of MTA out of the apex has been documented in the literature, and while not advocated, it has resulted in tooth survival. 10
Antibacterial (Basic pH)
MTA has a basic pH which gradually increases from initial mixing to reach a pH of 12.5 after four hours.11 This high pH is responsible for the antibacterial effects of MTA. This material has been shown to be effective against S. mutans, L. rhamnosus, L. paracasei, and P. gingivalius—all common microbes present in dental caries. 3 These properties are advantageous for a material used in vital pulp therapy as one may expect microbes to remain in dentin tubules despite conscious efforts to completely remove infected dentin.
Disadvantages
Long Setting Time
MTA sets in approximately two hours and 45 minutes. When the material is initially placed, its retention is purely mechanical. 1 Over time, however, a chemical bond forms with dentin. During this setting time, the material requires moisture, which can be problematic for many dental materials. Other potential problems for MTA include the following: 1) material can wash out due to prepping, and 2) MTA cannot be etched, rinsed, or bonded to. This means that most MTA must be temporized while it sets, or else the material must be covered with a moisture-tolerant product such as a glass ionomer cement before a moisture-intolerant substance such as composite resin can be used. Experience shows that pulpal therapy is more successful when a final restoration is placed at the time of treatment regardless of material placed.
Difficult Handling
Like using any material in dentistry, handling MTA involves a learning curve. 5 The learning experience is further complicated by the fact that MTA handles differently based on the amount of hydration involved in the mixing of the material. This author’s opinion is that the material handles similarly to sand. Wet MTA will handle like sand that has just been washed by a wave of the ocean, while dry MTA will resemble a sandcastle that has been baked by exposure to a warm day in the sun. Achieving the proper mixture is a key factor in ensuring the proper handling qualities calculated to match the clinical situation. The hydration properties can be advantageous when placing the material as an orthograde, root-end filling material in permanent teeth. If the material is too dry, it can be rehydrated to allow for apical advancement. 12
Cost
The cost of MTA can be a concern as practitioners look to keep office overhead low. The cost per use of material varies depending on the manufacturer and the type of packaging used. MTA requires moisture to set, and although uni-dose MTA products prevent the material from being exposed to humidity in the air, a single dose may contain more material than needed to treat a tooth, thus increasing waste. On the other hand, MTA that is stored in a multi-dose package, needs to be sealed from humidity to prevent the material from setting prior to use, another risk potentially leading to increased overhead costs.
MTA AS A BASE
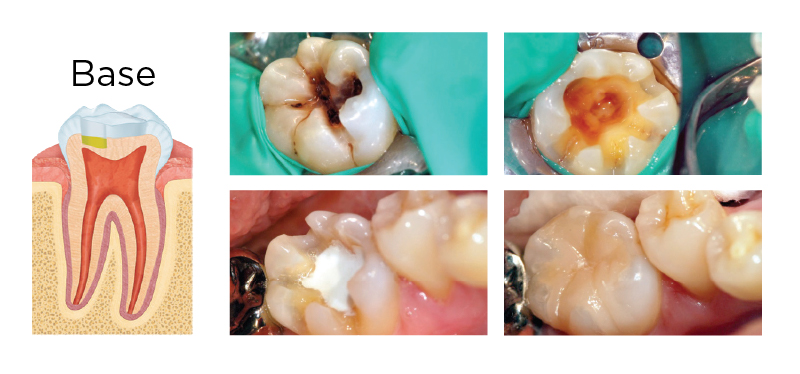
Fig. 1. A deep carious lesion (greater than two-thirds into dentin, upper left) was treated with an indirect pulp cap (base). Rubber dam isolation was utilized in case a pulp exposure occurred. After caries removal (upper right), 1.5 mm of SmartMTA was placed and allowed to set. Final restoration was completed with a Tesera resin inlay (bottom right).
MTA AS A DIRECT PULP CAP
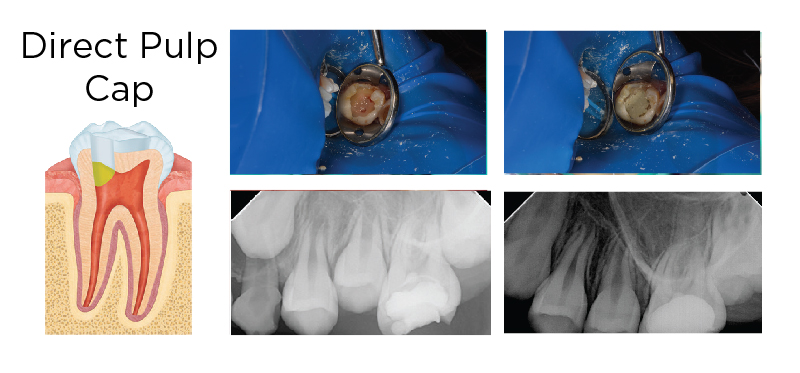
Fig. 2. A deep carious lesion was diagnosed with reversible pulpitis on a permanent molar treated with complete caries removal (upper left). The pulp was exposed and treated with sodium hypochlorite, and a layer of MTA was placed (upper right). The tooth was temporized (lower left) and restored with a resin composite (Premise) at a later date. At two-year recall, the tooth remains asymptomatic and shows no signs of apical pathology (lower right).
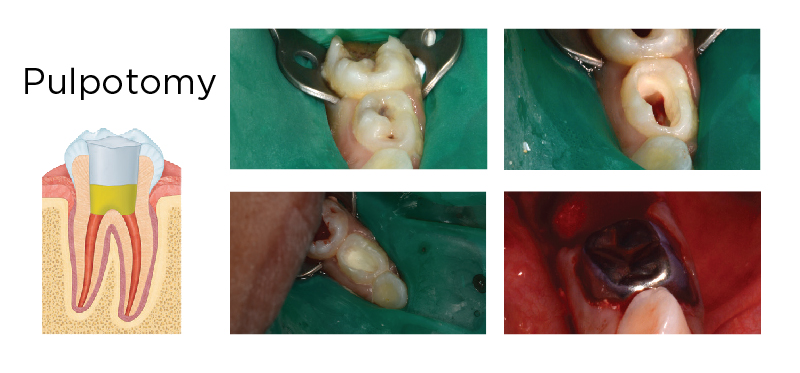
Fig. 3. MTA has been shown to have equivocal results as FC when used as a pulpotomy agent in primary teeth. Tooth #K and #L had deep carious lesions (upper left) and caries removal was completed for diagnosis (upper right). Tooth #K was hyperemic and extracted; tooth #L was treated with an MTA pulpotomy. After disinfection with sodium hypochlorite, MTA was adapted to the pulp covering the entire pulpal floor (lower left). The tooth was then restored with a full-coverage restoration (lower right).
Staining
The staining experienced in the use of MTA is attributed to bismuth oxide (an added radiopaquing agent) and sodium hypochlorite. 13 Sodium hypochlorite is a commonly used endodontic irrigant which dissolves organic matter, exhibits bactericidal and lubricating qualities, and is readily available. The staining can be reversed by bleaching after therapy is completed. However, this practice may be problematic in cases where there is no room in the pulp chamber. Newer formulations of calcium silicate products manifest less discoloration when they encounter sodium hypochlorite. 13
Conclusion
The field of pediatric dentistry continues to engage in an ongoing debate regarding the use of formocresol in primary tooth pulpotomies. Researchers debate formocresol’s toxicity and potential carcinogenicity, and the value of using MTA as a viable alternative.14,15 In scientific reviews, MTA has shown equivocal results compared to formocresol when used as a pulpotomy agent. 16,17 The material is a viable replacement for the formocresol pulpotomy. MTA is also superior to calcium hydroxide in direct pulp caps on permanent teeth and can be used to replace calcium hydroxide in the Cvek (partial) pulpotomy. 18,5 The versatility of the material in treating clinical scenarios of dental caries or trauma suggests that the material should hold an important place on the shelf of every pediatric dental office. The use of MTA in the treatment of primary and permanent teeth is soundly supported by the scientific literature. 5
IN SCIENTIFIC REVIEWS, MTA HAS SHOWN EQUIVOCAL RESULTS COMPARED TO FORMOCRESOL WHEN USED AS A PULPOTOMY AGENT.
THINGS TO CONSIDER WHEN CHOOSING AN MTA SYSTEM FOR YOUR PRACTICE:
- Setting Time
- Staining
- Cost
- Unit dose (contamination, setting if product is open)
- Handling (easy mix, place with familiar instrument [amalgam carrier])
- Dentin bridging (faster than CaOH, no bridge forms with Formo)
- Vital tissue is maintained (Formo mumi es tissue)
- Non-toxic/Biocompatible
- Antibacterial (pH)
- Bacterial entombment
- Diverse use in permanent and primary teeth (replaces multiple materials including CaOH and Formo)
REFERENCES
1. Torabinejad M, Hong C, McDonald F. Physical and Chemical Properties of a New Root-End Filling Material. J Endod. 1995;21(7):349–353.
2. Sakar N, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physiochemical Basis of the Biologic Properties of Mineral Trioxide Aggregate. J Endod. 2005;31(2):97–100.
3. Kim R, Kim M, Lee K, Lee D, Shin J. An in vitro evaluation of the antibacterial properties of three mineral trioxide aggregate (MTA) against five oral bacteria. Arc Oral Bio. 2015;60:1479–1502.
4. Yoo J, Chang S, Oh S, et al. Bacterial entombment by intratubular mineralization following orthograde mineral trioxide aggregate obturation: a scanning electron microscopy study. Int Jour Oral Sci. 2014(6):227–232.
5. Parirokh M, Torabinejad M. Mineral Trioxide Aggregtate: A Comprehnsive Literature Review — Part III: Clinical Applications, Drawbacks, and Mechnaism of Action. J Endod. 2010;36(3):400–413.
6. American Academy of Pediatric Dentistry. Guideline on Pulp Therapy for Primary and Immature Permanent Teeth. Pediatr Dent. 2014;36.
7. Ford T, Torabinejad M, Abedi H, Bakland L, Kariyawasam S. Using minteral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996;127(10):1491–1494.
8. De Rossi A, Bezerra Silva L, Gaton-Hernandez P, et al. Comparison of Pulpal Responses to Pulpotomy and Pulp Capping with Biodentine and Mineral Trioxide Aggregate in Dogs. J Endod. 2014;40:1362–1369.
9. Nowicka A, Lipski M, Parafiniuk M, et al. Response of Human Dental Pulp Capped with Biodentine and Mineral Trioxide Aggregate. J Endod. 2013;39(743–747).
10. Tahan E, Celik D, Er K, Tasdemir T. Effect of Unintentionally Extruded Mineral Trioxide Aggregate in Treatment of Tooth with Periradicular Lesion: A Case Report. J Endod. 2010;36(4):760–763.
11. Torabinejad M. Clinical Applications of Mineral Trioxide Aggregate. J Endod. 1999;25(3):197–205.
12. Al-Kahtani A, Shostad S, Scifferle R, Bhambhani S. In-Vitro Evaluation of Microleakage of an Orthograde Apical Plug of Mineral Trioxide Aggregate in Permanent Teeth with Simulated Immature Apices. J Endod. 2005;31(2):117–119.
13. Camilleri J. Staining Potential of Neo MTA Plus, MTA, Plus, and Biodentine Used for Pulpotomy Procedures. J Endod. 2015;41(7):1139–1145.
14. Casas M, Kenny D, Judd P, Johnston D. Do we still need formocresol in pediatric dentistry? J Can Dent Assoc. 2005;71(10):749–751.
15. Lewis B. The obsolescence of formocresol. J Calif Dent Assoc. 2010;38(2):102–107.
16. Stringhini E, Vitcel M, Oliveira L. Evidence of pulpotmy in primary teeth comparing MTA, calcium hydroxide, ferric sulphate, and electrosurgery with formocresol. Eur Arch paediatr Dent. 2015;16:303–312.
17. Coll J, Seale S, Vargas K, Marghalani A, Al Shamali S, Graham L. Primary Tooth Vital Pulp Therapy: A Systematic Review and Meta-analysis. Pediatr Dent. 2017;39(1):16–27.
18. Marques M, Wesselink P, Shemesh H. Outcome of Direct Pulp Capping with Mineral Trioxide Aggregate: A Prospective Study. J Endod. 2015;41(7):1023–1031.
Originally published in Shift Magazine, Spring 2017


